新英格兰:卡巴他赛与阿比特龙或恩杂鲁胺治疗转移性前列腺癌。前列腺癌是导致美国男性癌症死亡的第二大原因,在欧洲是癌症相关死亡的第三大原因。与雄激素信号靶向抑制剂(阿比特龙(abiraterone)和恩杂鲁胺enzalutamide))相比,卡巴他赛(cabazitaxel)对于转移性去势难治性前列腺癌的疗效和安全性尚不明确。
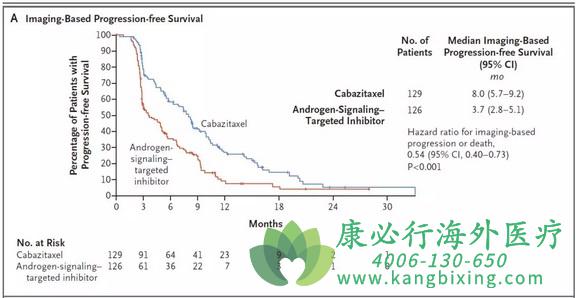
医学专家和教授们的报告。近日,新英格兰杂志发表了一项研究,该研究按1:1的比例将以前接受过多西他赛和雄激素信号靶向抑制剂(阿比特龙或恩杂鲁胺)的255名患者分为接受卡巴他赛(在25mg/m2体表面积剂量下,每3周静脉注射一次,每天加用泼尼松和粒细胞集落刺激因子)或其他雄激素信号靶向抑制剂(每天1000毫克阿比特龙+泼尼松或160毫克恩扎鲁胺)。该研究得主要终点是基于影像学的无进展生存期。该研究发现经经过中位期为9.2个月的随访后,卡巴 他赛组129名患者中有95名(73.6%)出现了影像学进展或死亡,而在126名接受雄激素信号靶向抑制剂的患者中有101名出现了影像学进展或死亡(80.2%) (风险比0.54;95%置信区间[CI],0.40-0.73;p<0.001)。卡巴他赛组的基于影像学的中位无进展生存期为8.0个月,雄激素信号靶向抑制剂组为3.7个月。卡巴他赛组的中位总生存期为13.6个月,雄激素信号靶向抑制剂组为11.0个月(死亡风险比0.64;95%置信区间,0.46-0.89;p=0.008)。卡巴他赛组的中位无进展生存期为4.4个月,雄激素信号靶向抑制剂组为2.7个月(进展或死亡的风险比,0.52;95%置信区间,0.40-0.68;p<0.001),前列腺特异性抗原反应分别为35.7%和13.5%(p<0.001),肿瘤反应分别为36.5%和11.5%(p=0.004)。不良反应事件发生率无统计学差异。该研究认为与阿比特龙或恩杂鲁胺相比,卡巴他赛治疗转移性前列腺癌显着改善了许多临床结果。a
摘要全文BACKGROUND
The efficacy and safety of cabazitaxel, as compared with an androgen-signaling–targeted inhibitor (abiraterone or enzalutamide), in patients with metastatic castration-resistant prostate cancer who were previously treated with docetaxel and had progression within 12 months while receiving the alternative inhibitor (abiraterone or enzalutamide) are unclear.
METHODS
We randomly assigned, in a 1:1 ratio, patients who had previously received docetaxel and an androgen-signaling–targeted inhibitor (abiraterone or enzalutamide) to receive cabazitaxel (at a dose of 25 mg per square meter of body-surface area intravenously every 3 weeks, plus prednisone daily and granulocyte colony-stimulating factor) or the other androgen-signaling–targeted inhibitor (either 1000 mg of abiraterone plus prednisone daily or 160 mg of enzalutamide daily). The primary end point was imaging-based progression-free survival. Secondary end points of survival, response, and safety were assessed.
RESULTS
A total of 255 patients underwent randomization. After a median follow-up of 9.2 months, imaging-based progression or death was reported in 95 of 129 patients (73.6%) in the cabazitaxel group, as compared with 101 of 126 patients (80.2%) in the group that received an androgen-signaling–targeted inhibitor (hazard ratio, 0.54; 95% confidence interval [CI], 0.40 to 0.73; P<0.001). The median imaging-based progression-free survival was 8.0 months with cabazitaxel and 3.7 months with the androgen-signaling–targeted inhibitor. The median overall survival was 13.6 months with cabazitaxel and 11.0 months with the androgen-signaling–targeted inhibitor (hazard ratio for death, 0.64; 95% CI, 0.46 to 0.89; P=0.008). The median progression-free survival was 4.4 months with cabazitaxel and 2.7 months with an androgen-signaling–targeted inhibitor (hazard ratio for progression or death, 0.52; 95% CI, 0.40 to 0.68; P<0.001), a prostate-specific antigen response occurred in 35.7% and 13.5% of the patients, respectively (P<0.001), and tumor response was noted in 36.5% and 11.5% (P=0.004). Adverse events of grade 3 or higher occurred in 56.3% of patients receiving cabazitaxel and in 52.4% of those receiving an androgen-signaling–targeted inhibitor. No new safety signals were observed.
CONCLUSIONS
Cabazitaxel significantly improved a number of clinical outcomes, as compared with the androgen-signaling–targeted inhibitor (abiraterone or enzalutamide), in patients with metastatic castration-resistant prostate cancer who had been previously treated with docetaxel and the alternative androgen-signaling–targeted agent (abiraterone or enzalutamide). (Funded by Sanofi; CARD ClinicalTrials.gov number, NCT02485691. opens in new tab.)
参考文献
De Wit R, De Bono J, Sternberg C N, et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer[J]. New England Journal of Medicine, 2019, 381(26): 2506-2518.
详情请访问 阿比特龙 https://www.kangbixing.com



 添加康必行顾问,想问就问
添加康必行顾问,想问就问